NEWS
See all articles >
Cecilia Marinova, MD, MBA
Managing Partner
Our vision
Our vision is to provide medical access to programs, diagnosis, treatment and medical solutions in areas of unmet medical needs.
Our commitment
Our commitmemt is lower risk market access scenario & cost effective business solutions.
We at Medasol are a creative and effective European team with a strong medical background and business experience.
We are focused on the best solution for market access and business entrance for specialized treatments, rare diseases and innovative drugs. We follow and cooperate with academics on their projects and help patient associations in articulating their needs. A virtual organisation around Medasol will support the launch of your product.
Medasol offers 3 effective commercial models. Business model solution is based on common preference for responsibility for processes and cost assignments:
1Commercialization via
Royalty Fees
2Commercialization via
Shared Responsibility
3Commercialization via
Consultancy Contract
We transform strategies into business results. We show the option, suggest the best solution and you choose.
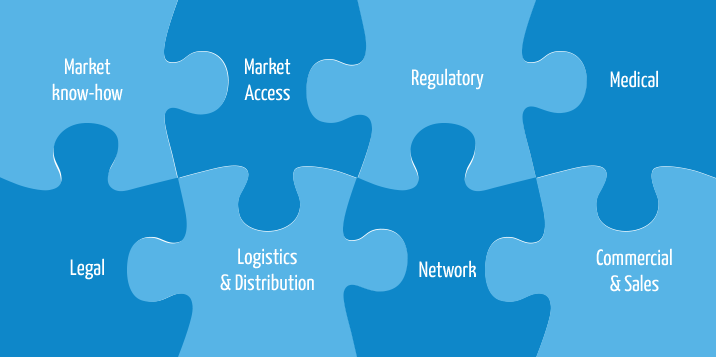
What stands behind our expertise:
We can reach your goals by the right plan, activities and cooperation.

Medasol offers the following services
Medasol s. r. o.
Drtinova 10
150 00 Praha 5
Registration address:
U poštovky 1269/2
150 00 Praha 5
+420 724 333 313